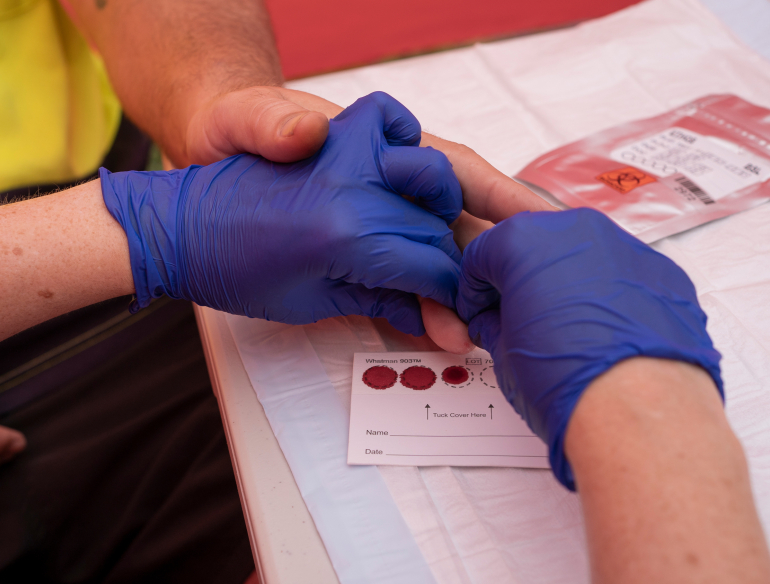
Low rates of hepatitis C virus (HCV) testing, diagnosis, and linkage to care are a major barrier to increasing treatment to achieve elimination targets. Currently a person usually attends multiple visits to a health practitioner for blood collection and need to wait for lab test results. This is further influenced by the lack of on-site blood collection (which may require off-site referral to a pathology centre) and among people who inject drugs, poor venous access.
Strategies to reduce HCV prevalence and incidence will require targeting of testing, linkage to care and treatment to people who have recently injected drugs. People with ongoing injecting drug use attend needle and syringe programs (NSP) to collect sterile needles and other injecting equipment. NSPs offer a unique opportunity to engage with PWID who may not be otherwise engaged in health services.
The TEMPO study is a multi-centre, practice-level, cluster randomised controlled trial to compare point-of-care HCV RNA testing, dried blood spot testing, and standard of care to enhance treatment uptake among people with HCV who have recently injected drugs attending needle and syringe programs. The purpose of the study is to compare point-of-care HCV RNA testing, dried blood spot (DBS) testing, and standard of care to enhance treatment uptake among people with HCV who have recently injected drugs attending NSPs. The aim is to determine the proportion of HCV RNA positive participants who initiate HCV treatment at 12 weeks following enrolment. We will also evaluate the cost-effectiveness, affordability, and long-term impact of scaled-up HCV RNA testing and HCV treatment in NSP services. Lastly, we will develop a scale-up plan, implementation tool-kit, HCV education/training program, and laboratory framework to facilitate national expansion of point-of-care HCV RNA testing.
Participants will be recruited from primary NSPs in Australia. Participants will receive either point-of-care HCV RNA testing, DBS collection and HCV RNA testing, or referral for testing through standard of care (most often venepuncture-based blood collection), depending on cluster randomisation. HCV RNA negative participants will have no further assessments of visits as part of the study. HCV RNA positive participants will be enrolled in an observational cohort and followed up at 3 months, 6 months and 1 year. Study questionnaires to assess medical history, risk, sexual behaviour, cost and time for travel to and from NSP, and health wellbeing assessment will be completed at each study visit.
This project will establish a framework for the provision of HCV care for people who inject drugs attending NSP services; a key priority population in the NSW Hepatitis C Strategy (2014–2020) and National Hepatitis C Strategy (2014–2017). Given the strong collaborative arrangements between stakeholders, this project has considerable potential to facilitate the translation of research outcomes into policy and practice in Australia and internationally, including influencing HCV testing and treatment guidelines and informing future jurisdictional, national, and international hepatitis strategies.
NSW Ministry of Health; South Western Sydney Local Health District; Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM); AIDS Council for NSW (ACON); NSW Users and AIDS Association (NUAA); Peer Based Harm Reduction (WA); Drug and Alcohol Services South Australia, Government of South Australia; Hepatitis SA; Hepatitis Queensland; Queensland Injectors Health Network (QuIHN); Department of Health, Northern Territory Government; Northern Territory AIDS and Hepatitis Council (NTAHC); St Vincent’s Health Network Sydney; Metro North Hospital and Health Service, Queensland Government; North Coast HIV and Related Programs, Mid North Coast Local Health District.
The TEMPO study is funded by NHMRC Partnership Grant, Gilead Sciences, Maridulu Budyari Gumal Sydney Partnership for Health, Education, Research and Enterprise (SPHERE), and South Western Sydney Local Health District. The GeneXpert device and cartridges have been provided in-kind by Cepheid.