First-line antiretroviral regimens are safe, effective and easily administered (one pill taken once daily). Even so, the annual failure rate is around 10-15% of treated patients. Second-line regimens have a number of limitations, disproportionately impacting resource-limited settings. Ideally, choice of the second-line regimen is guided by HIV drug resistance testing, however this is expensive and technically demanding. Drug resistance, previous treatment, and known toxicities mean individuals require a tailored regimen of second-line therapy where multiple pills are taken more than once a day. Unaddressed, the challenge of care for patients failing first-line antiretroviral therapy could profoundly and negatively affect the long-term effectiveness of treatment programs for HIV positive individuals and their communities.
The trial is designed to establish if two simplified combinations of antiretroviral drugs (dolutegravir with ritonavir boosted darunavir and dolutegravir with tenofovir and lamivudine or emtricitabine) are as safe and effective as the recommended World Health Organisation standard of care second-line regimens and, secondarily, to evaluate the simplified combinations against each other. The benefits of these novel combinations include a high barrier to resistance (negating the need for HIV drug resistance testing), the ability to co-formulate the drugs into a single pill requiring once daily dosing, cost savings as the simplified regimens would support task-shifting where prescriptions can be written by clinical nurses or physician assistants and simplification of supply chain and distribution by reducing the number of pills required.
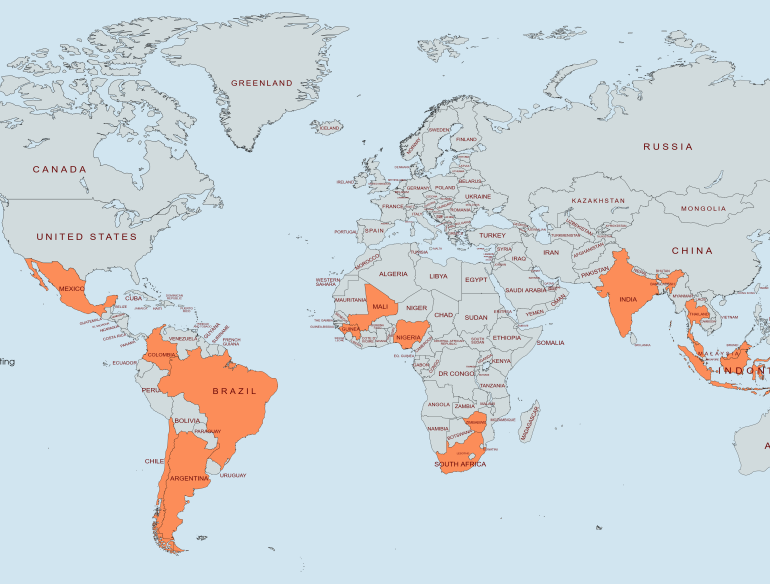
D²EFT is a randomised, open-label study recruiting participants from sites in Argentina, Brazil, Chile, Colombia, India, Indonesia, Guinea, Malaysia, Mali, Mexico, Nigeria, South Africa, Thailand and Zimbabwe. The design is based on the hypothesis that the simplified regimens will be non-inferior to SOC in terms of virologic control. The primary endpoint is the proportion of participants in each arm whose plasma viral load is <50 copies/mL at 48 weeks. The open label nature of the study allows routine care to be undertaken. A comparison of costs and estimates of cost-effectiveness between the treatment groups will be made utilising drug costs, health-care costs and quality of life measures.
The 48 primary analysis is expected in early 2023. Final week 96 analyses would be expected 12 months later.
The over-arching goal of the project is to provide the highest standard of evidence that will inform applicable treatment guidelines for second-line HIV therapy in resource-limited settings. It will either provide further validation for current recommendations or offer alternate approaches. If the latter, we would anticipate that the resulting recommendations would be implemented through the responsible agencies within defined geographical areas or countries. By incorporating diversity in our trial design we ensure the results are broadly applicable and generalisable to a similarly diverse set of clinical situations, making our findings directly translatable into the treatment of millions of people in resource-limited settings.
- Argentina: CICAL, Fundacion IBIS, Hospital Interzonal de Agudos San Juan de Dios, Hospital G de Agudos JM Ramos Mejia, Hospital Dr Diego Paroissien, CAICI
- Brazil: Instituto Nacional de Infectologia - Fiocruz
- Chile: Hospital San Borja-Arriaran
- Colombia: ASISTENCIA Cientifica De Alta Complejidad S.A.S.
- Guinea: Centre de traitementambulatoire de Donka ( Hopital de jour)
- India: CART CRS, VHS Hospital
- Indonesia: INA-RESPOND, Dr. Cipto Mangunkusumo Hospital, RSUP Dr. Wahidin, Sudirohusodo, Dr. Soetomo Hospital, Dr Sardjito Hospital
- Malaysia: University of Malaya Medical Centre, Hospital Pulau Pinang
- Mali: University of Sciences, Techniques and Technologies of Mali, University Clinical Research Center (UCRC)
- Mexico: Instituto Nacional de Ciencias Medicas y Nutriciòn Salvador Zubiran, Morales Vargas Centro de Investigacion SC, Hospital Civil de Guadalajara
- Nigeria: Institute of Human Virology, Nigeria (IHVN)
- South Africa: Desmond Tutu Health Foundation, Clinical HIV Research Unit (CHRU), Wits Health Consotium (Pty) Ltd, Perinatal HIV Research Unit (PHRU), Chris Hani Baragwanath Hospital
- Thailand: HIV-NAT (The HIV Netherlands Australia Thailand Research Collaboration), Thai Red Cross AIDS Research Centre, Srinagarind Hospital, Khon Kaen University, Bamrasnaradura Infectious Diseases Institute, Chiangrai Prachanukroh Hospital
- Zimbabwe: University of Zimbabwe Clinical Research Centre
Unitaid; National Health and Medical Research Council (NHMRC); National Institute of Allergy and Infectious Diseases (NIAID); ViiV Healthcare; Janssen Pharmaceuticals