Sydney, Australia – 20 March 2017: As a result of international research led by UNSW’s Kirby Institute, the U.S. Food and Drug Administration (FDA) has tentatively approved the use of a new fixed dose combination (FDC) of antiretroviral (ARV) drugs through the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) program, which will be available in developing countries at a substantially discounted price to current first line treatment.
The new FDC contains a lower dose (400mg) of efavirenz, one of the world’s most important components of first line HIV treatment, following confirmation from the Kirby Institute’sENCORE1trial that the lower dosage is just as effective and better tolerated than the previously recommended 600 mg daily dose.Data from the trial were published in two separate articles in The Lancet and The Lancet Infectious Disease in 2013 and 2014.
The research has served as the foundation for revisions to international treatment guidelines developed by the World Health Organization (WHO) that recommended 400mgof efavirenz as a valid treatment option in first line therapy.
Translation of these important findings into treatment programs was hampered by the absence of a pharmaceutical product that contained the lower dose of efavirenz in combination with other antiretroviral drugs. In collaboration with Mylan Pharmaceuticals and colleagues at the Clinton Health Access Initiative (CHAI), UNSW researchers can today confirm that a new product has been created and is now tentatively approved by the principal regulatory agency in the world. This will allow public sector procurement programs around the world to purchase and use this new medication. Because the product costs less due to the lower amount of efavirenz in the FDC, it is anticipated that more drug can be purchased and more people treated with the same amount of funding. Estimates of the relative saving to these international procurement programs could be up to US$150 million per year.
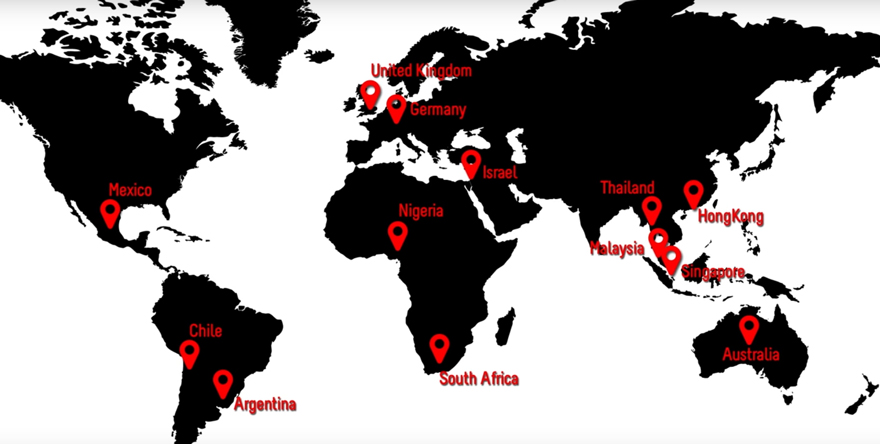
The trial was conducted in 13 countries involving 38 investigatoinal centres on six continents over a three year period.
“The direct translation of this body of research into international treatment guidelines and now its application in product development will change the way millions of people living with HIV receive treatment,” said Professor Sean Emery, Deputy Executive Dean and Research Dean at The University of Queensland, who led the study from the Kirby Institute. “The biggest impact of this development will be felt in low and middle-income countries, where stagnating foreign aid investment results in reduced access to antiretroviral therapy for those who need it.
“This is an extraordinary outcome,” said Professor David Cooper, Director of the Kirby Institute. It is a testament to the outstanding leadership of Sean Emery and the unwavering commitment of the project team, as well as investigators in Africa, Asia, Australia, Europe and Latin America and our other partners who worked tirelessly for many years to produce research findings that will have a direct and tangible positive impact on the health of millions of people around the world.”
The ENCORE1 study was funded by a $15 million grant from the Bill and Melinda Gates Foundation.
The Kirby Institute is a leading global research institute of UNSW Sydney, dedicated to the prevention and treatment of infectious diseases.
Media contact:
Laurie Legere, the Kirby Institute, +61 (0) 413 476 647 llegere@kirby.unsw.edu.au
Professor Sean Emery, The University of Queensland, +61 (7) 334 65305
More information:
https://www.youtube.com/watch?v=KcMzat9W6DQ
http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)62187-X/abstract
Header image:
Know your HIV status by Jon Rawlinson (CC BY 2.0).
Contact
Laurie Legere
Phone
0413 476 647
