The Kirby Institute at UNSW Sydney has received $2.2 million from the National Health and Medical Research Council, industry and other partners for a world-first study, called TEMPO, which will test a new intervention to scale-up hepatitis C testing and treatment among people who inject drugs attending needle and syringe programs.
The advent of simple, well-tolerated, direct-acting antiviral hepatitis C therapies with cure rates greater than 95% is one of the greatest medical advances in decades. However, low rates of hepatitis C testing, diagnosis, and linkage to care are major barriers to increasing treatment and eliminating hepatitis C infection in Australia, especially among people who inject drugs.
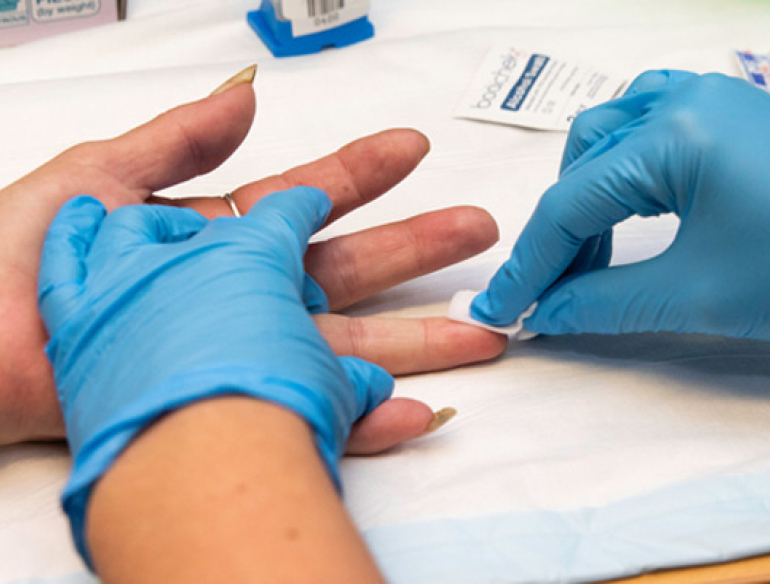
The TEMPO Partnership Project will conduct an innovative cluster randomised controlled trial to evaluate whether finger-stick hepatitis C testing, offer of same-day HCV therapy and peer-based support can enhance diagnosis, linkage to care and treatment among people who inject drugs attending needle and syringe programs.
In Australia, many people who inject drugs attend needle and syringe programs to collect sterile needles and other injecting equipment. Needle and syringe programs offer a unique opportunity to engage with people who inject drugs, a population who may not be engaged with other health services.
 Professor Jason Grebely
Professor Jason Grebely
“This development of a novel finger-stick test to diagnose hepatitis C in less than an hour has led to a paradigm shift, enabling same-day treatment for hepatitis C,” says Professor Jason Grebely, who is Chief Investigator on the project. “The TEMPO Study is one of the first studies globally to evaluate whether finger-stick hepatitis C testing, same-day treatment and peer-based support can enhance treatment uptake among people who inject drugs, making it incredibly innovative.”
Chief Investigators Professor Jason Grebely and Dr Tanya Applegate led the first international evaluations of this novel finger-stick point-of-care assay to detect active hepatitis C published last year in the Lancet Gastroenterology and Hepatology and in the Journal of Infectious Diseases.
“Australia is on-track to achieve hepatitis C elimination, but we need new and innovative strategies to enhance linkage to testing, care and treatment,” said Professor Gregory Dore, who is also a Chief Investigator on the project. “The findings from the TEMPO Project have the potential to change clinical practice, health policy, and the lives of people living with hepatitis C in Australia and globally.”
The project includes research to understand patient, provider and policy maker attitudes and potential barriers to point-of-care testing and treatment (led by Professor Carla Treloar at the Centre for Social Research, UNSW Sydney). It will also explore the cost-effectiveness, affordability and long-term impact of scaling up this approach to testing and treatment (led by Associate Professor Virginia Wiseman at the Kirby Institute, UNSW Sydney).
The TEMPO Study received $1,396,395 from an NHMRC partnership grant, and has support from Gilead Sciences, Cepheid and South Western Sydney Local Health District (Drug Health Services). The research will be conducted in partnership with the Centre for Social Research in Health (Professor Carla Treloar), the National Drug and Alcohol Centre (Professor Louisa Degenhardt and Dr Sarah Larney) at UNSW Sydney, and Kirketon Road Centre, South East Sydney Local Health District (Dr Phillip Read).
The study has also received support from Maridulu Budyari Gumal, The Sydney Partnerships in Health, Education and Enterprise (SPHERE), an innovative advanced health translation partnership between 14 leaders in health, education and medical research in NSW, including UNSW Sydney and stretching across four health services that treat 5 million people. This includes seed funding from the Infection, Immunity and Inflammation (Triple-I) Clinical Academic Group, which is part of the SPHERE partnership.
Other study partners include: ACON, ASHM, Cepheid, DASSA, Gilead Sciences, Hepatitis NSW, Hepatitis QLD, Hepatitis SA, Mid North Coast LHD, NSW Health, NT Health, NT AIDS and Hepatitis Council, NUAA, Peer Based Harm Reduction WA, QLD Health – Biala, Queensland Injectors Health Network, South East Sydney LHD, St Vincent’s Hospital, South West Sydney LHD, WA Health.