In November 2017, the US Clinical Practice Guidelines in Oncology were updated to list pomalidomide, an oral immune regulator, as the preferred regimen for relapsed Kaposi’s sarcoma (KS), based on a U.S. study led by the Kirby Institute’s Dr Mark Polizzotto, now head of the Therapeutic and Vaccine Research Program.
The study evaluated pomalidomide as a treatment regimen in patients with symptomatic KS in people with and without HIV, to establish whether HIV status affected the patients’ responses to the treatment. Dr Polizzotto and a team of researchers in the US including Dr Robert Yarchoan, Chief of the HIV and AIDS Malignancy Branch at the National Cancer Institute’s Center for Cancer Research, found that pomalidomide was well tolerated and active in KS regardless of HIV status and responses were rapid. Patients’ self-reported outcomes also improved.
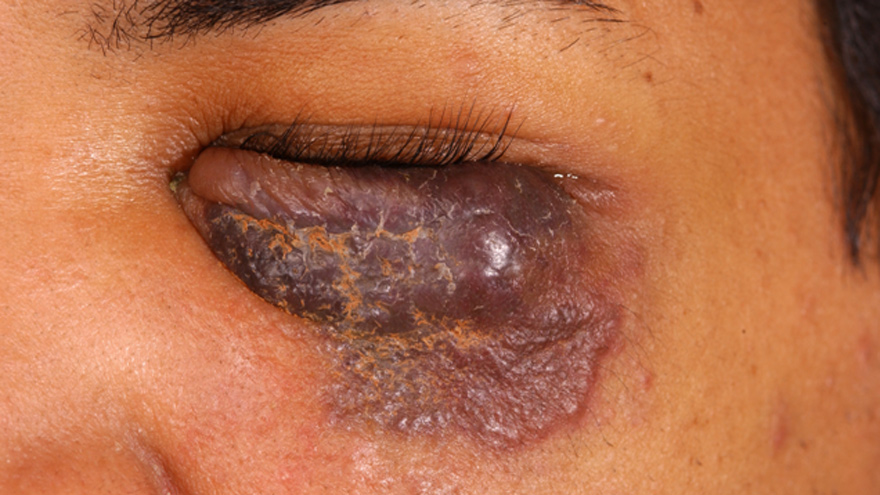
Kaposi’s sarcoma eyelid lesion. Photo courtesy of National Cancer Institute.
“This is the first change to the KS recommendation in over a decade, and will have the potential to address key unmet clinical needs for people living with KS,” says Dr Polizzotto. “It’s this type of work that’s rewarding as a researcher. We’re seeing our research translate into real-world changes to clinical guidelines.”
Dr Polizzotto’s study also provided the scientific rationale for a Kirby Institute study called SPACE which is currently underway. SPACE evaluates whether pomalidomide is effective in clearing high grade HPV-associated anogential lesions, before they progress and develop into anal cancer. There is currently no proven effective treatment for HPV-associated anal lesions, and rates of anal cancer are increasing in our population. If successful, this approach could ultimately allow early intervention and so offer a new approach to cancer prevention for individuals at the highest risk of developing HPV-associated cancers.