A clinical trial across 12 countries will test a potential COVID-19 treatment, using antibodies purified from plasma taken from people who have recovered from the disease.
Researchers from the Kirby Institute at UNSW Sydney together with colleagues in the United States and Europe are leading a multinational COVID-19 clinical trial funded by the United States National Institutes of Health (NIH), announced this month by Dr Anthony Fauci, Director of the U.S. National Institute of Allergy and Infectious Diseases.
The trial, called INSIGHT 013 or ITAC (Inpatient Treatment with Anti-Coronavirus Immunoglobulin), will test ‘hyperimmune immunoglobulin’ treatments in individuals hospitalised with COVID-19.
“Hyperimmune immunoglobulins are antibodies purified from plasma taken from people who have recovered from COVID-19. For ITAC, antibodies from up to 50 recovered individuals are pooled in the final product, which is designed to enhance the immune response to the virus and block its entry into cells,” says Associate Professor Mark Polizzotto, who is protocol chair of the ITAC trial.
The ITAC trial will be conducted at more than 60 sites across 12 countries. It is a randomised, double blinded, controlled platform trial of 500 patients, which will compare the effectiveness of hyperimmune immunoglobulin to placebo. All patients will receive standard-of-care therapies including remdesivir, an anti-viral drug shown to shorten recovery times in COVID-19 in another NIH-sponsored trial.
Four companies are collaborating to provide anti-coronavirus hIVIG for the trial: Emergent BioSolutions; Grifols S.A.; CSL Behring; and Takeda Pharmaceuticals.
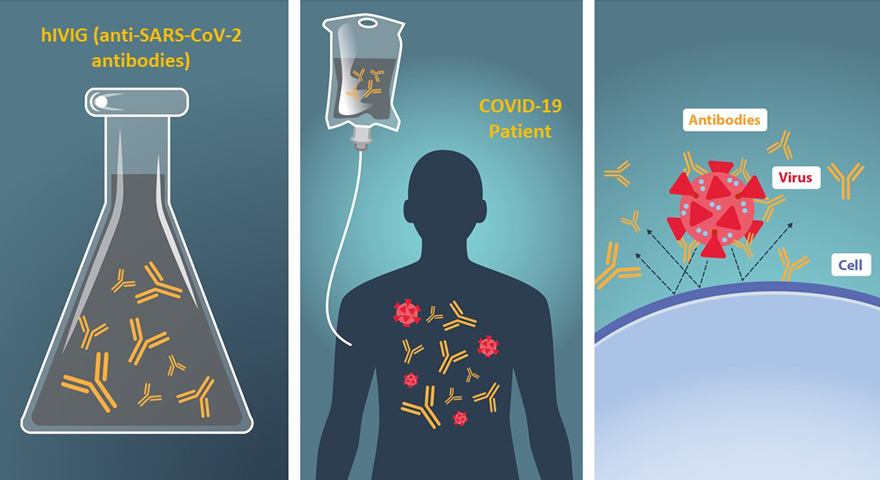 How can antibodies help? hIVIG contains antibodies from people who have recovered from an infection. Antibodies are made by your body to help fight disease. When germs, like the SARS-CoV-2 virus that causes COVID-19, enter your body, your immune system makes antibodies to fight that germ. We hope these antibodies can help stop the virus from getting into your cells and help you get better faster from COVID-19.
How can antibodies help? hIVIG contains antibodies from people who have recovered from an infection. Antibodies are made by your body to help fight disease. When germs, like the SARS-CoV-2 virus that causes COVID-19, enter your body, your immune system makes antibodies to fight that germ. We hope these antibodies can help stop the virus from getting into your cells and help you get better faster from COVID-19.
“This trial is a world-first in a number of ways,” said A/Prof. Polizzotto. “It’s the first time we’re testing the efficacy of this promising treatment in patients with COVID-19. It is also remarkable that four major pharmaceutical companies have come together to participate together in a single clinical trial. This exceptional, global scientific collaboration involving the public and private sector is a coordinated response to the seriousness of COVID-19.”
ITAC is being conducted by the INSIGHT network, a partnership of five institutions across the United States, Europe and Australia coordinated by the University of Minnesota. The Kirby Institute will coordinate trial implementation at sites in Asia, parts of Africa and the Middle East, and Latin America.
Recruitment has begun, with results expected later in 2020.
Australian ‘INSIGHT’ from HIV is facilitating a rapid COVID-19 response
ITAC is one of a number of COVID-19 treatment and observational trials launched by the INSIGHT network since the COVID-19 pandemic began. The unprecedented pace of trial development, shortening a process that usually take years to a matter of months, reflects years of preparation by network leaders.
INSIGHT (the International Network of Strategic Initiatives in Global HIV Trials) was established over 20 years ago in response to the HIV pandemic, and has since conducted pivotal trials in HIV and other viral infections including influenza.
“INSIGHT was conceived by colleagues at five universities along with the U.S. National Institutes of Health. Among those leaders were the Kirby Institute’s Professor David Cooper (who passed away in March 2018) and Professor Sean Emery (now the Senior Vice Dean Research at UNSW Medicine), so Australian leadership in these global clinical trials goes right back to the beginning,” said A/Prof. Polizzotto.
“The strength of INSIGHT is its global reach. This allows us to rapidly answer critical questions about treatments for COVID-19, ensuring we can offer the trial to patients wherever there is an outbreak. Evaluating treatments in a diverse range of populations, including those who are not normally represented in clinical trials, ensures the trial treatments are applicable to all those who need them.
“For infectious diseases like HIV, influenza and now COVID-19, there are regional variations in both the disease itself, and the health systems in which it’s treated. A gold-standard ‘successful treatment’ needs to be effective across a range of settings,” said A/Prof. Polizzotto.
“The true strength of INSIGHT is its diversity. It brings together study sites in low, middle, and high-income countries, and resources them effectively to find a treatment that will save lives.”
While Australia is playing a lead role in the conduct of these trials, there are currently no Australian sites for these trials due to the low COVID-19 numbers.
Media contact: Lucienne Bamford, Kirby Institute UNSW Sydney, 0432 894 029, lbamford@kirby.unsw.edu.au
About the Kirby Institute
The Kirby Institute is a leading global research institute dedicated to the prevention and treatment of infectious diseases. Established in 1986 in response to the then emerging HIV epidemic, the Institute now contributes to knowledge on a broad range of diseases, including viral hepatitis and sexually transmissible infections.
About INSIGHT
INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) has been funded to conduct HIV treatment trials since 2006 by the National Institute of Allergy and Infectious Diseases, a component of the National Institutes of Health.
Since then, INSIGHT's scope has broadened and now the network also conducts several influenza studies. Our mission is to define optimal strategies for the management of HIV and other infectious diseases through a global clinical research network. INSIGHT conducts studies worldwide.