The care cascade for chronic hepatitis B (CHB) diagnosis, treatment and monitoring in Australia remains suboptimal. Major gaps exist at every level of intervention with only 62% of Australians living with CHB diagnosed, 17% accessing guideline based care and 9% on treatment. These gaps are exacerbated by inequalities in access to care by region and population. Cheap hepatitis B virus (HBV) viral load tests that provide immediate results at the clinic would help link people to care, especially for those living in remote and resource limited settings and help reach National Hepatitis B targets.
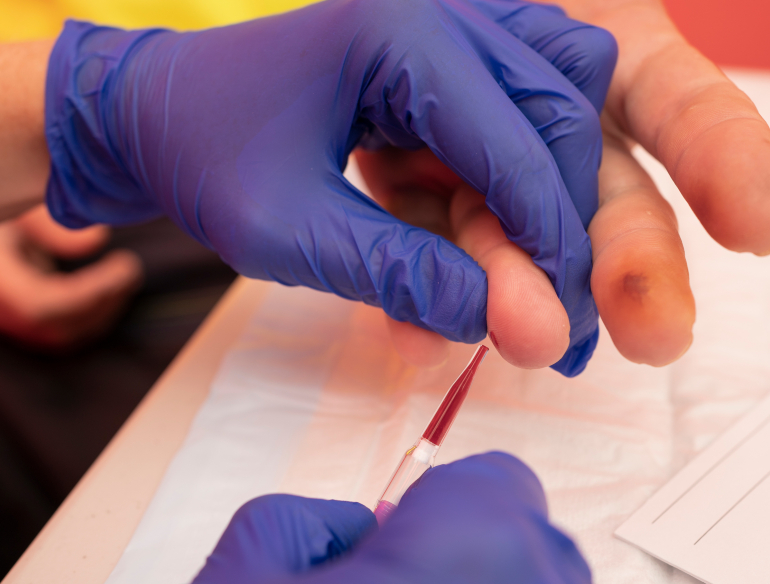
Quantitative HBV DNA tests currently require the collection of venous whole blood, which must be transported to centralised laboratories for testing. As a result, access to HBV DNA testing requires multiple visits at significant expense or is simply unavailable for many people living in remote or resource-limited settings. This clinical research project will assess the performance of a novel fingerstick HBV viral load within a network of Sydney HBV clinical sites. We will adapt the test to directly use fingerstick blood instead of processed whole blood; and measure its performance as a point-of-care test and in dried blood spots. We will assess the acceptability and potential uptake of such tests through patient-provider surveys.
The study will be conducted in two phases among patients attending 6 clinics in the inner-city, South Eastern, South Western and Western parts of Sydney. There will be a total of 300 participants recruited, phase 1 will recruit 60 participants and phase 2 will recruit 240 participants.
The Third National Hepatitis B strategy 2018–2022 sets ambitious targets including 80% of people living with CHB diagnosed, 50% receiving care and 20% on treatment. This project directly addresses several Priority Areas for Action within this strategy, including:
- Improving targeted guideline-based testing of priority populations
- Providing current, innovative and effective hepatitis B testing, vaccination and care
- Supporting equitable access to programs and services with a focus on innovative models of service delivery
- Strengthening links between service providers to better engage people living with or at risk of HBV with care.
We aim in achieving >80% of HBV diagnosis, engagement and treatment targets set in the Third Australian HBV strategy.
The project will provide robust evidence to support future studies to assess the feasibility, performance, acceptability, impact, and cost effectiveness of this novel assay among affected populations in remote and resource limited settings. Further the project will establish a framework for the conduct of future studies aimed at improving the cascade of care for people living with CHB and continue to build on our demonstrated excellence in conducting high quality implementation research among marginalised communities and remote Aboriginal communities.
Blacktown Hospital; St Vincent's Hospital; Liverpool Hospital; Prince of Wales Hospital; Westmead Hospital.
Australian Government Department of Health.